How Does Reverse Osmosis Work?
Water is an important element of life. However, due to organic, inorganic and biological reasons, it is getting polluted day-by-day. Drinking polluted water can affect your health and lead to diarrhea, skin rashes, typhoid etc. To keep such problems at bay, one of the most effective solutions is to use an RO water purifier. No doubt, that using an RO water purifier is the best solution, but many of us don’t know the working process. So, in this blog, we discuss in detail the step-by-step process describing how RO water purifier works and how each process helps in removing impurities to deliver pure and healthy drinking water.
What is Reverse Osmosis?
Reverse osmosis (RO) is a process that uses a semipermeable membrane to remove ions, molecules, and larger particles from a solution. The semipermeable membrane consists of a thin layer of polymers capable of rejecting the target material by applying pressure, whereas the rest of the water can pass through unhindered.
How does a RO (reverse osmosis) system work?
Reverse osmosis (RO) is a process that uses pressure to force water through a semipermeable membrane. The pressure creates a concentration gradient, driving water molecules through the membrane and leaving behind all dissolved solids.
The membranes used in reverse osmosis systems are designed to allow water molecules to pass through while retaining larger particles and dissolved solids. Reverse osmosis systems use pressure to create a concentration gradient across the membrane such that water flows from the high-concentration to the low-concentration side.
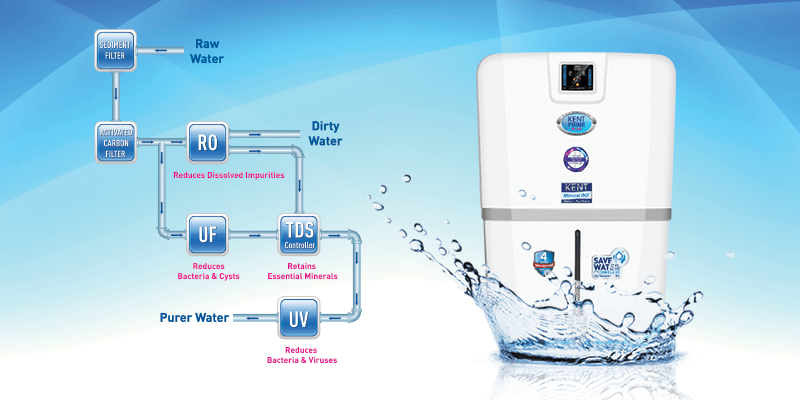
4 Step Purification Process of RO Water Purifier
An RO water purifier comes in a combination of sediment, carbon filter, RO membrane, UV lamp, UF membrane, and post-carbon filter. Let’s take a closer look at how these filters work.
Step 1. Pre-Filters
Pre-filter helps in removing larger particles such as sand silt, dirt and other sediments from water. The pre-filter helps in protecting the RO membrane that gets clogged as a result of exposure to sediment and chlorine. The carbon filter helps in removing toxic compounds such as pesticides and is even effective enough in removing bad odour from water.
Step 2. Reverse Osmosis Membrane
The next filter on our list is the RO membrane. Water is forced through the semi-permeable membrane in order to block the minute impurities. The membrane is a synthetic plastic material that stops sodium, chlorine, and even the larger molecules such as urea, bacteria, and viruses. The reverse osmosis drinking water systems also help in removing lead, arsenic, copper, chromium, selenium, fluoride etc.
Step 3. UV Lamp
RO water purifier also comes with a UV lamp that helps in disinfecting bacteria from the water by killing all the harmful pathogens present in the water. The high-power UV ray destroys the illness-causing microorganisms by attacking their genetic core, thereby eliminating their ability to reproduce. The ultimate work of a UV lamp is to destroy 99.99% of harmful microorganisms from the water, thereby making it safe for consumption.
Step 4. Ultrafiltration
The next process of filtration that the RO purifier has is UF. It is a type of hollow fibre membrane through which water is forced to pass leaving the impurities behind and delivering pure and clean water.
Step 5. Post Filter (Activated Carbon Filter)
Before the water finally gets filled in the storage tank, it passes through the post-carbon filter. The filter helps in removing any remaining contaminants that slip through the membrane and make it completely pure and fit for consumption.
Along with these filters, RO water purifier comes added with TDS controller that helps in retaining all the essential natural minerals in the purified water. Some of the RO water purifiers come with Save Water Technology that employs a computer-controlled process. The technology aid in recovering more than 50% water as purified water resulting in less water wastage. Besides, the RO wastewater can be used for different domestic purposes and even for watering the plant.

KENT’s All New ‘Zero Water Wastage Technology’
KENT’s Zero Water Wastage Technology ensures no wastage of water while the purification process is on, unlike other RO filters. Let’s check out how does KENT Zero Water Wastage Technology function:
1. Multistage Purification Process
Multistage Purification Technology helps in removing dissolved impurities from the water making it usable. It helps in removing impurities like rust, arsenic, pesticides and fluorides etc. and kills bacteria and viruses, thus making the water safe for consumption.
2. Zero Water Wastage Technology
KENT’s Zero Water Wastage Technology gives pure water, safe for consumption, while using the Reverse Osmosis process. But unlike the conventional methods of purification, KENT’s Zero Water Wastage Technology ensures that rejected water is sent back to the overhead tank with an internal pump, resulting in no wastage of water. One can reuse the water for other household chores.
3. Retains Essential Minerals
KENT’s Zero Water Wastage Technology helps in keeping the essential minerals remain intact while purifying the dissolved impurities like rust, arsenic, calcium and magnesium.
The Bottom Line
We hope you understood the step-by-step method of Reverse Osmosis water purification process. This is one of the most effective solutions that not only helps in removing impurities from water but also delivers clean and healthy drinking water. Bring home the best RO water purifier today!





Dear Manufacturer,
I would like to know, how TDS controller would maintain essential minerals.
Whether it will maintain arsenic or lead how it will be ensured.
Dear Sir,
Thanks for writing to us. Regarding your query, we would inform you that KENT RO water purifiers are based on patented Mineral RO technology that uses double Purification followed by UV/UF Purification that removes even dissolved impurities such as chemicals, bacteria, viruses and salts and retains essential natural minerals in purified water using the TDS Controller, thereby providing pure and safe drinking water. Please call us at 9582123456 for more details and information. Alternatively, you may visit the below link: https://www.kent.co.in/water-purifiers/ro/ You may also share your contact details at onlinecare@kent.co.in Our experts will get in touch with you.
Regards,
Team Kent
Information given herein is useful.
Would appreciate if the company arranges pre water testing and then recommending their matching water purifier to the customers accordingly. I am also interested in buying one piece.
Hi,
Thanks for showing interest in our products. We would be happy to help you. Request you to please share your contact number and location details at onlinecare@kent.co.in so that our experts will assist you better. You may also call us at 9582123456 for product details and more information. Alternatively, you may visit the below link: https://www.kent.co.in/water-purifiers/ro/
Regards,
Team Kent
Wow a great information on commercial RO plant. Read the whole blog and found it very informative as kent is doing a great work in water industry.
thank you for your valuable feedback. This means a lot to us. Please feel free to contact us in case you need any further assistance. Regards, Team Kent
Good information about how to reverse osmosis work?
Hi Aditya,
Regarding your query, we would inform you that KENT RO water purifiers are based on patented Mineral RO technology that uses double purification to combine Reverse Osmosis (RO) and Ultraviolet (UV) / Ultra Filtration (UF) in a multistage filtration process that removes even dissolved impurities such as chemicals, bacteria, viruses and salts and retains essential natural minerals in purified water using the TDS Controller, thereby providing safe. Please call us at 9582123456 for product details and more information. Alternatively, you may visit the below link:https://www.kent.co.in/water-purifiers/ro/. Let us know if you any other query.
Regards,
Team Kent