pH level in drinking water- Why do you need to be concerned?
Water, an essential element of life, contains a number of dissolved salts, impurities, and minerals. The water that we receive has four properties which are pH, buffering capacity, salinity, and general hardness. Among these four properties, the pH level of water is of great importance. Danish chemist, S.P.L Sorensen, was the first person to introduce the concept of pH scale and its impact on health. Though we are concerned about water purification, many of us are not aware of the importance of maintaining the pH of drinking water. In this blog, we discuss the importance of pH levels in water and their impact on health.
What is pH level?
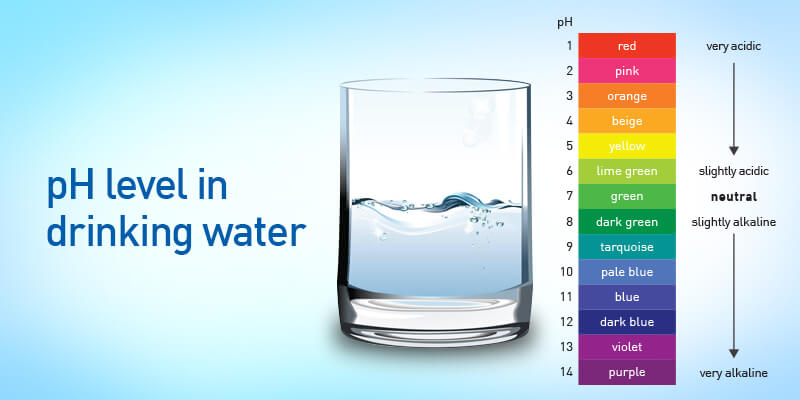
pH measures the level of acidity and alkalinity in different kinds of liquids including water. The range of pH scales varies between 0-14 with 7.0 being the normal pH level. Anything less than 7 is considered acidic, whereas anything higher than 7 is considered to be alkaline. Food items such as yogurt, cheese, or fish are more acidic, whereas vegetables such as beets, bell peppers, and kale are alkaline. In addition to the food items, it is equally important to check the pH value of drinking water. Drinking acidic water can lead to a number of problems such as weight gain, slow immune response as well as susceptibility to diseases.
Relation between pH and Health
1. pH level and digestive system
Our digestive system produces hydrochloric acid which helps in digesting food without harming the stomach. However, the presence of excess hydrochloric acid can lead to problems such as indigestion leading to pain, and irritation.
2. Enzymes and pH level
Enzymes play an important role in breaking down carbohydrates that we consume. Enzymes cause chemical reactions to break down protein into amino acids, carbohydrates into glucose, and fats into fatty acids. If the pH level for a particular enzyme is too high or low, the normal functions of breaking down proteins and fats may take a longer time.
3. pH and Tooth Decay
The bacteria present in our mouths break down foods that contain sugar into acids. These acids help in lowering the pH level in the mouth. If the pH level falls below 5.5, it can lead to tooth decay. The reason is the acids become strong enough to destroy the enamel of teeth, leading to corrosion and tooth decay.
pH level and Water
Pure water, which is rarely available, has the right pH level. Factors such as bedrock, and soil composition are some of the factors that affect the pH scale of water. The pH value of drinking water needs to be monitored closely to ensure that the pH level doesn’t exceed 7. Water with a pH level of less than 7 has a corrosive quality and a metallic taste. This implies water may contain iron, copper, zinc, and from the plumbing fixtures. Water with a high alkaline level forms scale or precipitate on the plumbing fixture and also lead to a number of health problems.
How to maintain the pH level in water?

One of the easy ways to maintain the best pH level in water is to use KENT Alkaline Water Filter Pitcher. The water filter pitcher from KENT provides clean, germ-free water and at the same time maintains the pH of drinking water. The highly durable food-grade plastic and smart design make it easy to use and store the alkaline pitcher. If you want to find out more about KENT Alkaline Water Filter Pitcher, click here.